Lesson: Carbon and its Compounds
Question:1
What
would be the electron dot structure of carbon dioxide which has the formula ?
Solution:
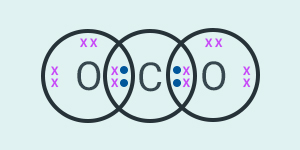
Question:2
What
would be the electron dot structure of a molecule of sulphur which is made up
of eight atoms of sulphur?
(Hint
The eight atoms of sulphur are joined together
in the form of a ring.)
Solution:
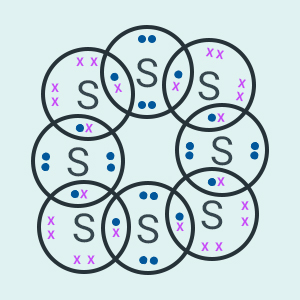
Question:3
How
many structural isomers can you draw for pentane?
Solution:
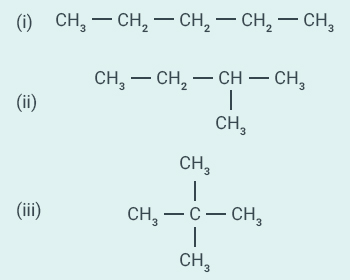
Question:4
What
are the two properties of carbon which lead to the huge number of carbon
compounds we see around us?
Solution:
The following
are the features due to which carbon atoms can form a large number of
compounds:
·
Catenation
Carbon can form bond with other carbon atoms.
·
Tetravalency
The valency of carbon is four. It means that one
carbon atom can form bonds with four different atoms. Even if one of these four
atoms is changed, a new compound is formed.
Question:5
What
will be the formula and electron dot structure of cyclopentane?
Solution:
The formula of
cyclopentane is .
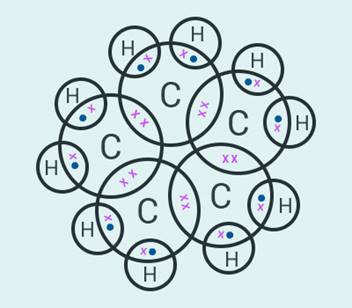
Question: 6
Draw
the structures for the following compounds.
(a) Ethanoic acid
(b) Bromopentane*
(c) Butanone
(d) Hexanal.
*Are
structural isomers possible for bromopentane?
Solution:
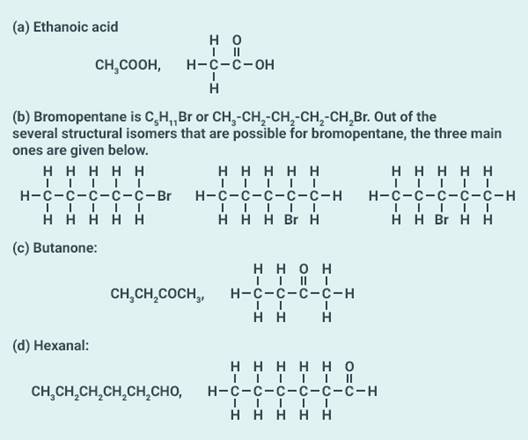
Question: 7
How
would you name the following compounds?
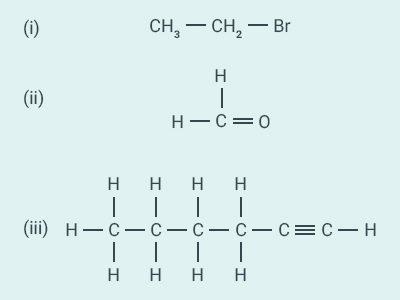
Solution:
(i) Bromoethane
(ii) Methanal (formaldehyde)
(iii)
Hexyne
Question: 8
Why
is the conversion of ethanol to ethanoic acid an oxidation reaction?
Solution:
In this reaction, one oxygen atom is added to
ethanol to form ethanoic acid. Hence, it
is an oxidation reaction.
Question: 9
A
mixture of oxygen and ethyne is burnt for welding.
Can
you tell why a mixture of ethyne and air is not used?
Solution:
When a mixture of oxygen and ethyne is
burnt for welding, the following reaction takes place:
Due to the presence of oxygen, complete
combustion takes place.
The
air, however, contains limited supply of oxygen. So, when ethyne is burnt in
air, it results in incomplete combustion.
Question: 10
How
would you distinguish experimentally between an alcohol and a carboxylic acid?
Solution:
An alcohol
and a carboxylic acid can be distinguished on the basis of their reaction with
carbonates and hydrogen carbonates.
On reacting with an acid, a carbonate or
a hydrogen carbonate give . gas turns lime water milky.
An alcohol, however, does not react
with carbonates and hydrogen carbonates.
Question: 11
What
are oxidising agents?
Solution:
The substances
that give oxygen or replace hydrogen in a chemical reaction are called
oxidising agents.
Question: 12
Would
you be able to check if water is hard by using a detergent?
Solution:
No, it is not possible to check if water is hard by
using detergent as detergents form lather with both hard and soft water.
Question: 13
People use a variety of methods to wash clothes.
Usually after adding the soap, they ‘beat’ the clothes on a stone, or beat it
with a paddle, scrub with a brush or the mixture is agitated in a washing
machine. Why is agitation necessary to get clean clothes?
Solution:
One end of a soap
molecule is hydrophobic and the other is hydrophilic. The hydrophobic end
attaches to the dirt particles and forms a cluster called micelle.
The hydrophilic
end remains attached to water.
When the cloth
is agitated, these micelles get dispersed in water due to which the soap water
becomes dirty and the clothes get cleaned.





