Lesson: Acids, Bases, and Salts
Question:1
A
solution turns red litmus blue; its pH is likely to be:
(a)
1
(b)
4
(c)
5
(d)
10
Solution:
(d)
Question:2
A
solution reacts with crushed egg-shells to give a gas that turns lime-water
milky. The solution contains:
(a)
NaCl
(b)
HCl
(c)
LiCl
(d)
KCl
Solution:
(b)
Question:3
10
mL of a solution of NaOH is found to be completely neutralised by 8 mL of a given
solution of HCl. If we take 20 mL of the same solution of NaOH, the amount HCl
solution (the same solution as before) required to neutralise it will be:
(a)
4 mL
(b)
8 mL
(c)
12 mL
(d)
16 mL
Solution:
(d)
Question:4
Which
one of the following types of medicines is used for treating indigestion?
(a)
Antibiotic
(b)
Analgesic
(c)
Antacid
(d)
Antiseptic
Solution:
(c)
Question: 5
Write
word equations and then balanced equations for the reaction taking place when
(a)
Dilute sulphuric acid reacts with zinc granules.
(b)
Dilute hydrochloric acid reacts with magnesium ribbon.
(c)
Dilute sulphuric acid reacts with aluminium powder.
(d)
Dilute hydrochloric acid reacts with iron filings.
Solution:
a)
(b)
(c)
(d)
Question: 6
Compounds
such as alcohols and glucose also contain hydrogen but are not categorised as
acids. Describe an activity to prove it.
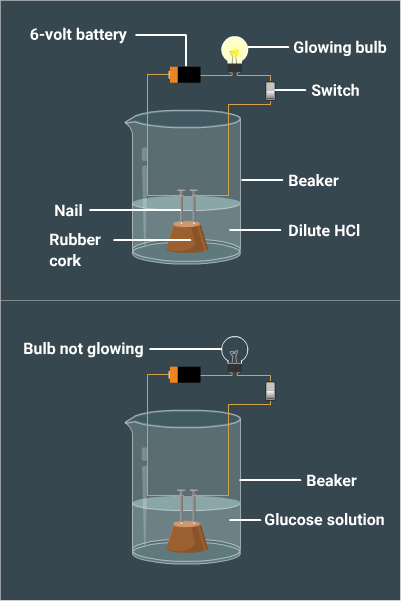
Solution:
·
Take
two nails fitted on a
cork and kept in a 100-mL beaker.
·
Connect the nails to two terminals of a 6-volt
battery through a bulb and a switch.
·
Pour
dilute HCl in the beaker such that the nails dip into it.
·
Switch
on the current.
·
Repeat
the same
experiment with glucose solution and alcohol solution.
Observations:
It is observed that the bulb glows in
the HCl solution and does not glow in the glucose and alcohol solution.
Result:
HCl dissociates into ions. These ions conduct
electricity in the solution resulting in glowing of the bulb.
Alcohol and glucose solutions do not dissociate into ions and
therefore they do not conduct electricity.
That is why, though alcohols and glucose contain hydrogen, they
are not categorised as acids.
Question: 7
Why does distilled water not conduct electricity,
whereas rain water does?
Solution:
Distilled water is
neither acidic nor basic in nature. So it does not dissociate into ions. Therefore, it does not conduct electricity.
Rainwater becomes
acidic due to its reaction with acidic gases. Like any other acid, rain water
conducts electricity due to the formation of ions.
Question: 8
Why do acids not show acidic behaviour in the
absence of water?
Solution:
An acid ionises on dissolving in water to
produce hydrogen ions. It is the presence of these ions that makes it behave
like an acid.
An acid will not show acidic behaviour in
the absence of water.
Question: 9
Five solutions A, B, C, D and E when tested with
universal indicator showed pH as:
4, 1, 11, 7 and 9, respectively. Which solution is:
(a) Neutral?
(b) Strongly alkaline?
(c) Strongly acidic?
(d) Weakly acidic?
(e) Weakly alkaline?
Arrange the pH in increasing order of hydrogen-ion
concentration.
Solution:
(a)
(b)
(c)
(d)
(e)
The
pH can be arranged in the increasing order of the concentration of hydrogen
ions as: 11 < 9 < 7 < 4 < 1.
Question: 10
Equal lengths of magnesium ribbons are taken in test
tubes A and B. Hydrochloric acid ( ) is added to test tube A, while acetic acid ( ) is added to test tube B. Amount and concentration
taken for both the acids are same. In which test tube will the fizzing occur
more vigorously and why?
Solution:
is a stronger
acid than . It produces hydrogen gas at a faster speed due to
which the fizzing occurs. Therefore, the fizzing will occur vigorously in test
tube A, in which hydrochloric acid ( ) is added.
Question: 11
Fresh milk has a pH of 6. How do you think the pH
will change as it turns into curd? Explain your answer.
Solution:
The pH of milk
is 6. As it turns into curd, the pH will reduce because curd is more acidic in
nature than milk.
Question: 12
A milkman adds a very small amount of baking soda
to fresh milk.
(a) Why does he shift the pH of the fresh milk from
6 to slightly alkaline?
(b) Why does this milk take a long time to set as
curd?
Solution:
(a) In an alkaline
condition, milk does not set as curd easily. The milkman shifts the pH of the
fresh milk from 6 to slightly alkaline to keep it fresh for a longer time.
(b) When baking
soda is added, milk becomes more basic than usual. Hence, acids produced to set
the milk as curd are neutralized by the base added to milk. Therefore, it takes
a longer time for the milk to set as curd.
Question: 13
Plaster of Paris should be stored in a
moisture-proof container. Explain why?
Solution:
Plaster of Paris (POP) should be stored in a
moisture-proof container because Plaster of Paris absorbs water (moisture)
to form a hard solid called gypsum.
Question: 14
What is a neutralisation reaction? Give two
examples.
Solution:
A reaction in which an acid reacts with a base to
give a salt and water is called neutralisation reaction. Given below are
two examples of neutralisation reaction:
1.
2.
Question: 15
Give two important uses of washing soda and baking
soda.
Solution:
Washing soda
is used:
(a) In glass,
soap, and paper industries.
(b) To remove
permanent hardness of water.
Baking soda is used:
(a) In the
food industries, mainly in the bakeries, to make the bread or cake fluffy.
(b) In soda-acid
fire extinguishers.
