Lesson: Periodic Classification of Elements
Question: 1
Upto
which element, the Law of Octaves was found to be applicable:
(a)
Oxygen
(b)
Calcium
(c)
Cobalt
(d)
Potassium
Solution:
(b)
Question:2
According
to Mendeleev’s Periodic Law, the elements were arranged in the periodic table
in the order of:
(a)
Increasing atomic number
(b)
Decreasing atomic number
(c)
Increasing atomic masses
(d)
Decreasing atomic masses
Solution:
(c)
Question:3
In Mendeleev’s
Periodic Table, gaps were left for the elements to be discovered later.
Which
of the following elements found a place in the periodic table later?
(a)
Germanium
(b)
Chlorine
(c)
Oxygen
(d)
Silicon
Solution:
(a)
Question: 4
Which
of the following statement(s) about the Modern Periodic Table is(are) incorrect:
(i)
The elements in the Modern Periodic Table are arranged on the basis of their
decreasing atomic number.
(ii)
The elements in the Modern Periodic Table are arranged on the basis of their
increasing atomic masses.
(iii)
Isotopes are placed in adjoining group(s) in the Periodic Table.
(iv)
The elements in the Modern Periodic Table are arranged on the basis of their
increasing atomic number.
(a)
(i) only
(b)
(i), (ii) and (iii)
(c)
(i), (ii) and (iv)
(d)
(iv) only
Solution:
(b)
Question: 5
Which
of the following statements about the Modern Periodic Table are correct:
(a)
It has 18 horizontal rows known as Periods
(b)
It has 7 vertical columns known as Periods
(c)
It has 18 vertical columns known as Groups
(d)
It has 7 horizontal rows known as Groups
Solution:
(c)
Question:6
Which
of the given elements A, B, C, D and E with atomic number 2, 3, 7, 10 and 30
respectively belong to the same period?
(a)
A, B, C
(b)
B, C, D
(c)
A, D, E
(d)
B, D, E
Solution:
(b)
Question:7
The
elements A, B, C, D and E have atomic number 9, 11, 17, 12 and 13 respectively.
Which
pair of elements belong to the same group?
(a)
A and B
(b)
B and D
(c)
A and C
(d)
D and E
Solution:
(c)
Question: 8
Where
would you locate the element with electronic configuration 2,8 in the Modern
Periodic Table?
(a)
Group 8
(b)
Group 2
(c)
Group 18
(d)
Group 10
Solution:
(c)
Question: 9
An
element which is an essential constituent of all organic compounds belongs to:
(a)
Group 1
(b)
Group 14
(c)
Group 15
(d)
Group 16
Solution:
(b)
Question; 10
Which of the following is the outermost shell for
elements of period 2?
(a) K shell
(b) L shell
(c) M shell
(d) N shell
Solution:
(b)
Question: 11
Which one of the following elements exhibit maximum
number of valence electrons?
(a) Na
(b) Al
(c) Si
(d) P
Solution:
(d)
Question: 12
Which
of the following gives the correct increasing order of the atomic radii of O, F
and N?
(a)
O, F, N
(b)
N, F, O
(c)
O, N, F
(d)
F, O, N
Solution:
(d)
Question: 13
Which
among the following elements has the largest atomic radii?
(a)
Na
(b)
Mg
(c)
K
(d)
Ca
Solution:
(c)
Question 14
Which
of the following elements would lose an electron easily?
(a)
Mg
(b)
Na
(c)
K
(d)
Ca
Solution:
(c)
Question: 15
Which
of the following elements does not lose an electron easily?
(a)
Na
(b)
F
(c)
Mg
(d)
Al
Solution:
(b)
Question: 16
Which of the following are the characteristics of
isotopes of an element?
(i) Isotopes of an element have same atomic masses
(ii) Isotopes of an element have same atomic number
(iii) Isotopes of an element show same physical
properties
(iv) Isotopes of an element show same chemical
properties
(a) (i), (iii) and (iv)
(b) (ii), (iii) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Solution:
(d)
Question: 17
Arrange
the following elements in the order of their decreasing metallic character:
Na,
Si, Cl, Mg, Al
(a)
Cl > Si > Al > Mg > Na
(b)
Na > Mg > Al > Si > Cl
(c)
Na > Al > Mg > Cl > Si
(d)
Al > Na > Si > Ca > Mg
Solution:
(b)
Question: 18
Arrange the following elements in the order of
their increasing non-metallic character Li, O, C, Be, F
(a) F < O < C < Be < Li
(b) Li < Be < C < O < F
(c) F < O < C < Be < Li
(d) F < O < Be < C < Li
Solution:
(b)
Question: 19
What
type of oxide would Ekaaluminium form?
(a)
(b)
(c)
(d)
Solution:
(c)
Question: 20
Three
elements B, Si and Ge are:
(a)
Metals
(b)
Non-metals
(c)
Metalloids
(d)
Metal, non-metal and metalloid respectively
Solution:
(c)
Question: 21
Which
of the following elements will form an acidic oxide?
(a)
An element with atomic number 7
(b)
An element with atomic number 3
(c)
An element with atomic number 12
(d)
An element with atomic number 19
Solution:
(a)
Question: 22
The element with atomic number 14 is hard and forms
acidic oxide and a covalent halide.
To which of the following categories does the
element belong?
(a) Metal
(b) Metalloid
(c) Non-metal
(d) Left-hand side element
Solution:
(b)
Question: 23
Which one of the following depicts the correct
representation of atomic radius(r) of an atom?
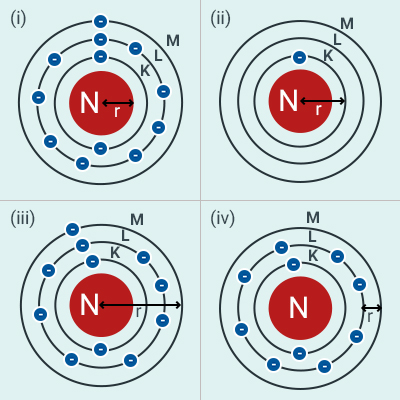
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Solution:
(b)
Question: 24
Which one of the following does not increase while
moving down the group of the periodic table?
(a) Atomic radius
(b) Metallic character
(c) Valence
(d) Number of shells in an element
Solution:
(c)
Question 25
On moving from left to right in a period in the periodic table, the size
of the atom.
(a) Increases
(b) Decreases
(c) Does not change appreciably
(d) First decreases and then increases
Solution:
(b)
Question: 26
Which of the following set of elements is written in
order of their increasing metallic character?
(a) Be Mg Ca
(b) Na Li K
(c) Mg Al Si
(d) C O N
Solution:
(a)
Question: 27
The three elements A, B and C with similar
properties have atomic masses X, Y and Z respectively.
The mass of Y is approximately equal to the average
mass of X and Z. What is such an arrangement of elements called as?
Give one example of such a set of elements.
Solution:
The arrangement
of these elements is known as Döbereiner’s triad.
Example:
Lithium, Sodium and Potassium
Question: 28
Elements have been arranged in the following
sequence on the basis of their increasing atomic masses.
F, Na, Mg, Al, Si, P, S, Cl, Ar, K
(a) Pick two sets of elements which
have similar properties.
(b) The given sequence represents which
law of classification of elements?
Solution:
(a) (i) F and Cl
(ii) Na and K.
(b) Newland’s
law of octaves
Question: 29
Can the following groups of elements be classified
as Dobereiner’s triad?
(a) Na, Si, Cl
(b) Be, Mg, Ca
Atomic mass of Be 9; Na 23; Mg 24; Si 28; Cl 35; Ca
40
Explain by giving reason.
Solution:
(a) No, because all these elements do not have
similar properties although the
atomic mass of
silicon is approximately average of the atomic masses of
sodium (Na) and
chlorine (Cl).
(b) Yes,
because they have similar properties and the mass of magnesium (Mg) is
roughly the
average of the atomic mass of Be and Ca. Therefore, they form a
Dobereiner’s triad.
Question: 30
In Mendeleev’s Periodic Table the elements were
arranged in the increasing order of their atomic masses. However, cobalt with
atomic mass of 58.93 amu was placed before nickel having an atomic mass of
58.71 amu. Give reason for the same.
Solution:
This was done to ensure that elements with similar
chemical properties were placed in the same group.
Question: 31
“Hydrogen occupies a unique position in Modern
Periodic Table”. Justify the statement.
Solution:
Hydrogen
has one electron in its outermost orbit. Alkali metals too have the same number
of electrons in its outermost orbit. Hydrogen resembles alkali metals as well
as halogens. Though hydrogen is placed along with alkali metals in group 1,
hydrogen is not included in the discussion of group 1 metals.
Thus,
hydrogen occupies a unique position in the modern periodic table and is studied
separately.
Question: 32
Write the formulae of chlorides of Eka-silicon and
Eka-aluminium, the elements predicted by Mendeleev.
Solution:
Question: 33
Three elements A, B and C have 3, 4 and 2 electrons
respectively in their outermost shell.
Give the group number to which they belong in the
Modern Periodic Table.
Also, give their valences.
Solution:
|
Element |
Group No. |
Valence |
|
A |
Group-13 |
3 |
|
B |
Group-14 |
4 |
|
C |
Group-2 |
2 |
Question: 34
If
an element X is placed in group 14, what will be the formula and the nature of
bonding of its chloride?
Solution:
; Covalent bonding.
Question: 35
Compare the radii of two species X and Y.
Give reasons for your answer.
(a) X has 12 protons and 12 electrons
(b) Y has 12 protons and 10 electrons
Solution:
Radius of Y is
lesser than that of X because Y is the ion formed when X loses 2 electrons.
Question: 36
Arrange the following elements in increasing order
of their atomic radii.
(a) Li, Be, F, N
(b) Cl, At, Br, I.
Solution:
(a) F < N
< Be < Li
(b) Cl < Br < I < At
Question: 37
Identify and name the metals out of the following
elements whose electronic configurations are given below.
(a) 2, 8, 2
(b) 2, 8, 1
(c) 2, 8, 7
(d) 2, 1.
Solution:
Here (a), (b) and (d) are metals.
(a) Magnesium
(b) Sodium
(d) Lithium.
Question: 38
Write the formula of the product formed when the
element A (atomic number 19) combines with the element B (atomic number 17).
Draw its electronic dot structure. What is the nature of the bond formed?
Solution:
The formula of the product will be AB.
The electron dot structure will be as given below:
The elements A and B will form an ionic bond.
A = K (Potassium)
B = Cl (Chlorine)
Question: 39
Arrange the
following elements in the increasing order of their metallic character.
Mg, Ca, K, Ge, Ga.
Solution:
Question: 40
Identify the elements with the following property
and arrange them in increasing order of their reactivity:
(a) An element which is a soft and reactive metal
(b) The metal which is an important constituent of
limestone
(c) The metal which exists in liquid state at room
temperature
Solution:
(a) Na or K
(b) Ca
(c) Hg
Question: 41
Properties of the elements are given below.
Where would you locate the following elements in
the periodic table?
(a) A soft metal stored under kerosene
(b) An element with variable (more than one)
valency stored under water.
(c) An element which is tetravalent and forms the
basis of organic chemistry
(d) An element which is an inert gas with atomic
number 2
(e) An element whose thin oxide layer is used to
make other elements corrosion
resistant by the process of “anodising”.
Solution:
(a)
Sodium (Na) in Group 1 and Period 3 or Potassium
(K) in Group 1 and Period 4
(b)
Phosphorus (P) in Group 15 and Period 3
(c)
Carbon (C) in Group 14 and Period 2
(d)
Helium (He) in Group 18 and Period 1
(e)
Aluminium (Al) in Group 13 and Period 3
Question: 42
An element is placed in 2nd Group and 3rd Period of
the Periodic Table, burns in presence of oxygen to form a basic oxide.
(a) Identify the element
(b) Write the electronic configuration
(c) Write the balanced equation when it burns in
the presence of air
(d) Write a balanced equation when this oxide is
dissolved in water
(e) Draw the electron dot structure for the
formation of this oxide
Solution:
(a) An element in 2nd group and 3rd period is magnesium
(Mg).
(b)
The
electronic configuration of magnesium is 2, 8, 2.
(c) When magnesium burns in the presence of air,
magnesium oxide is formed. The balanced reaction would be:
(d) When magnesium oxide dissolves in water, magnesium
hydroxide is formed. The balanced reaction would be:
(e) The electron dot structure is:
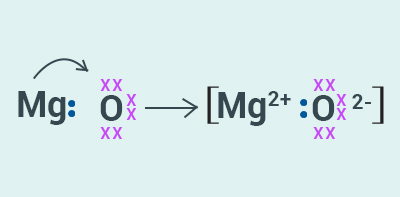
Question: 43
An element X
(atomic number 17) reacts with an element Y (atomic number 20) to form a
divalent halide.
(a) Where in the
periodic table are elements X and Y placed?
(b) Classify X
and Y as metal(s), non-metal(s) or metalloid(s)
(c) What will
be the nature of oxide of element Y?
(d)Identify the
nature of bonding in the compound formed
(e) Draw the
electron dot structure of the divalent halide.
Solution:
(a) The atomic number of X is 17. So, its electronic
configuration would be 2, 8, 7. This element would therefore belong to Group 17
and 3rd period. The atomic number of Y is 20. So, its electronic
configuration would be 2, 8, 8, 2. This element would belong to Group 2 and 4th
period.
(b) The electronic configuration of X is 2, 8, 7. It
will be easier for X to gain an electron and complete its octet during
formation of bonds. Thus, X is a non-metal.
(c) The electronic configuration of Y is 2, 8, 8, 2. It
will be easier for Y to lose 2 electrons and complete its octet during
formation of bonds. Thus, Y is a metal.
(d) Oxide of Y will be basic in nature. Also, the oxide
will have ionic bond.
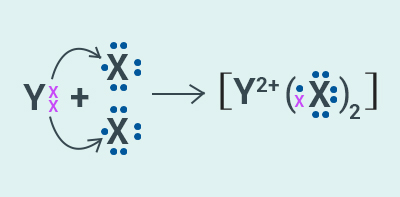
Question: 44
Atomic number
of a few elements are given below 10, 20, 7, 14:
(a) Identify the elements
(b) Identify the Group number of these elements in the
Periodic Table
(c) Identify the Periods of these elements in the
Periodic Table
(d) What would be the electronic configuration for each
of these elements?
(e) Determine the valency of these elements
Solution:
The table below
gives the elements with atomic number 10, 20, 7, 14, their electronic
configuration, group, period and valency:
|
Atomic
number |
Element |
Electronic
configuration |
Group |
Period |
Valency |
|
10 |
Neon
(Ne) |
2, 8 |
18 |
2 |
0 |
|
20 |
Calcium
(Ca) |
2, 8, 8,
2 |
2 |
4 |
2 |
|
7 |
Nitrogen
(7) |
2, 5 |
15 |
2 |
3 |
|
14 |
Silicon
(Si) |
2, 8, 4 |
14 |
3 |
4 |
Question: 45
Complete the
following cross word puzzle given below:
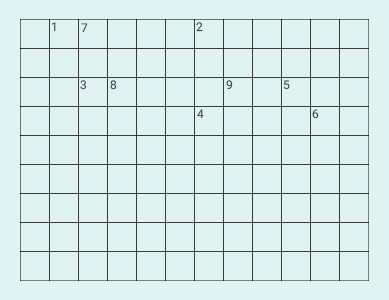
Across:
(1) An element
with atomic number 12.
(2) Metal used
in making cans and member of Group 14.
(3) A lustrous
non-metal which has 7 electrons in its outermost shell.
Down:
(4) Highly
reactive and soft metal which imparts yellow colour when subjected to
flame and is
kept in kerosene.
(5) The first
element of second Period
(6) An element
which is used in making fluorescent bulbs and is second member
of Group 18 in
the Modern Periodic Table
(7) A
radioactive element which is the last member of halogen family.
(8) Metal which
is an important constituent of steel and forms rust when exposed
to moist air.
(9) The first
metalloid in Modern Periodic Table whose fibres are used in making
bullet-proof
vests.
Solution:

Question: 46
(a) In this ladder symbols of elements are jumbled up.
Rearrange these symbols of elements in the increasing order of their atomic number
in the Periodic Table.
(b) Arrange them in the order of their group also.
Solution:
(a) H, He, Li,
Be, B, C, N, O, F, Ne, Na, Mg, Al, Si, P, S, Cl, Ar, K, Ca
(b) Group 1 H, Li, Na, K
Group 2 Be, Mg, Ca
Group 13 B, Al
Group 14 C, Si
Group 15 N, P
Group 16 O, S
Group 17 F, Cl
Group 18 He, Ne, Ar
Question: 47
Mendeleev predicted
the existence of certain elements not known at that time and named two of them
as Eka-silicon and Eka-aluminium:
(a) Name the elements which have taken the place of
these elements
(b) Mention the group and the period of these elements
in the Modern Periodic Table.
(c) Classify these elements as metals, non-metals or
metalloids
(d) How many valence electrons are present in each one
of them?
Solution:
(a) The elements discovered after Mendeleev’s
prediction are germanium (Ge) and gallium (Ga).
(b) Germanium (Ge) belongs to group 14 and period 4 while
gallium (Ga) belongs to group 13 and
period 4.
(c) Germanium (Ge) is a metalloid and gallium (Ga) is a
metal.
(d) Germanium (Ge) has 3 valance electrons and gallium (Ga)
has 4.
Question: 48
i.
Electropositive
nature of the element(s) increases down the group and
decreases across the period
ii. Electronegativity of the element decreases down the
group and increases
across the period
iii. Atomic size increases down the group and decreases
across a period
(left to right)
iv. Metallic character increases down the group and
decreases across a period.
On the basis of
the above trends of the Periodic Table, answer the following
about the
elements with atomic numbers 3 to 9.
(a) Name the most electropositive element among
them
(b) Name the most electronegative element
(c) Name the element with smallest atomic size
(d) Name the element which is a metalloid
(e) Name the element which shows maximum valency.
Solution:
(a) Lithium
(b) Fluorine
(c) Fluorine
(d) Boron
(e) Carbon
Question: 49
An element X which is a yellow solid at room
temperature shows catenation and allotropy.
X forms two oxides which are also formed during the
thermal decomposition of ferrous sulphate crystals and are the major air
pollutants.
(a) Identify the element X
(b) Write the electronic configuration of X
(c) Write the balanced chemical equation for the
thermal decomposition of ferrous
sulphate crystals?
(d) What would be the nature (acidic/basic) of
oxides formed?
(e) Locate the position of the element in the Modern
Periodic Table.
Solution:
(a)
The element described here is sulphur (atomic
no. 16).
(b)
The electronic configuration of sulphur is 2,
8, 6.
(c)
When heated, ferrous sulphate gives, ferric
oxide, sulphur dioxide and sulphur trioxide. The balanced equation for the
reaction is:
(d)
The oxides of sulphur are acidic in nature.
(e)
Sulphur belongs to the 3rd period
and group 16 of the Modern Periodic Table.
Question: 50
An element X of group 15 exists as diatomic
molecule and combines with hydrogen at 773 K in presence of the catalyst to
form a compound, ammonia which has a characteristic pungent smell.
(a) Identify the element X. How many valence electrons
does it have?
(b) Draw the electron dot structure of the diatomic
molecule of X.
(c) What type of bond is formed in it?
(d) Draw the electron dot structure for ammonia and
what type of bond is formed in it?
Solution:
(a) The element described here is nitrogen. It has atomic
number 7. Its electronic configuration is 2, 5. It has 5 valence electrons.
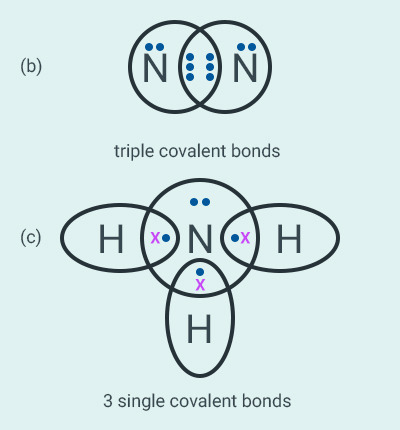
Question: 51
Which group of elements could be placed in Mendeleev’s Periodic Table without disturbing the
original order? Give reason.
Solution:
Noble gases could be placed in Mendeleev’s periodic
table without disturbing the original order.
According to Mendeleev’s classification, the
properties of elements are the periodic function of their atomic masses. Noble gases
are inert and could be placed in a separate group without disturbing the
original order.
Question: 52
Give an account of the process adopted by Mendeleev for the classification of elements. How
did he arrive at “Periodic Law”?
Solution:
In 1869, Mendeleev made a remarkable contribution
to the classification of elements. When Mendeleev selected his work, 63
elements were known. He examined the relationship between the atomic masses of
the elements and their physical and chemical properties. Among the chemical
properties, the compounds of these elements with oxygen and hydrogen were
studied (formation of oxides and hydrides). He then took 63 cards and on each
he wrote down the properties of one element. He sorted out the elements with
similar properties and paired the cards together. He observed that, elements
with similar properties recur at regular intervals or periodically. He also observed
that most of the elements get a place in the periodic table and was arranged in
order of their increasing atomic masses.
On this basis, Mendeleev formulated a periodic law,
which states that “the properties of elements are the periodic function of their
atomic masses”.