Lesson: Carbon and its Compounds
Question: 1
Carbon exists in the atmosphere in the
form of:
(a) Carbon monoxide only
(b) Carbon monoxide in traces and
carbon dioxide
(c) Carbon dioxide only
(d) Coal
Solution:
b
Question:2
Which of the following statements are
usually correct for carbon compounds?
(i) are good conductors of electricity
(ii) are poor conductors of electricity
(iii) have strong forces of attraction
between their molecules
(iv) do not have strong forces of
attraction between their molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Solution:
d
Question:3
A molecule of ammonia ( )
has:
(a) Only single bonds
(b) Only double bonds
(c) Only triple bonds
(d) Two double bonds and one single
bond
Solution:
a
Question: 4
Buckminsterfullerene is an allotropic
form of:
(a) Phosphorus
(b) Sulphur
(c) Carbon
(d) Tin
Solution:
c
Question: 5
Which of the following are correct
structural isomers of butane?
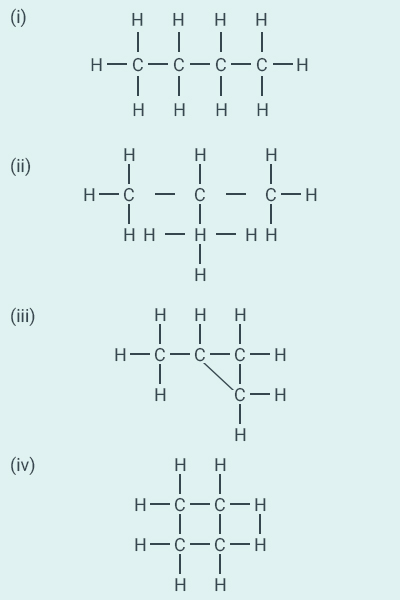
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Solution:
c
Question:6
In the above given reaction, alkaline acts as:
(a) Reducing agent
(b) Oxidising agent
(c) Catalyst
(d) Dehydrating agent
Solution:
b
Question:7
Oils on treating with hydrogen in the
presence of palladium or nickel catalyst form fats.
This is an example of:
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction
Solution:
a
Question: 8
In which of the following compounds, OH is the
functional group?
(a) Butanone
(b) Butanol
(c) Butanoic acid
(d) Butanal
Solution:
b
Question: 9
The soap molecule has a:
(a) Hydrophilic head and a hydrophobic
tail
(b) Hydrophobic head and a hydrophilic
tail
(c) Hydrophobic head and a hydrophobic
tail
(d) Hydrophilic head and a hydrophilic
tail
Solution:
a
Question:
10
Which of the following is the correct
representation of electron dot structure of nitrogen?
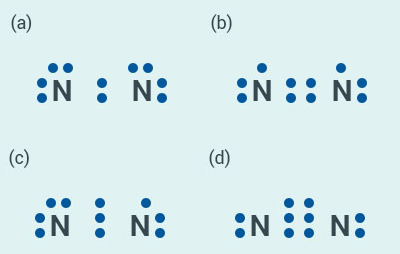
Solution:
d
Question: 11
Structural formula of ethyne is:
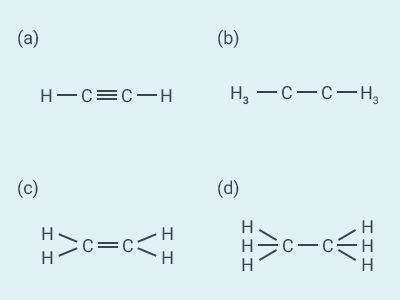
Solution:
a
Question: 12
Identify the unsaturated compounds from
the following:
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii)
Solution:
d
Question:
13
Chlorine reacts with saturated
hydrocarbons at room temperature in the:
(a) Absence of sunlight
(b) Presence of sunlight
(c) Presence of water
(d) Presence of hydrochloric acid
Solution:
b
Question:
14
In the soap micelles:
(a) The ionic end of soap is on the
surface of the cluster while the carbon chain is in the interior of the
cluster.
(b) Ionic end of soap is in the
interior of the cluster and the carbon chain is out of the cluster.
(c) Both ionic end and carbon chain are
in the interior of the cluster
(d) Both ionic end and carbon chain are
on the exterior of the cluster
Solution:
a
Question:
15
Pentane has the molecular formula . It has:
(a) 5 covalent bonds
(b) 12 covalent bonds
(c) 16 covalent bonds
(d) 17 covalent bonds
Solution:
c
Question:
16
Structural formula of benzene is:
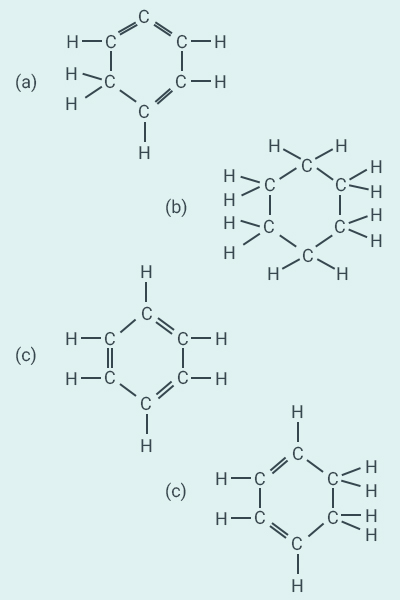
Solution:
c
Question:
17
Ethanol reacts with sodium and forms
two products.
These are:
(a) Sodium ethanoate and hydrogen
(b) Sodium ethanoate and oxygen
(c) Sodium ethoxide and hydrogen
(d) Sodium ethoxide and oxygen
Solution:
c
Question: 18
The correct structural formula of
butanoic acid is:
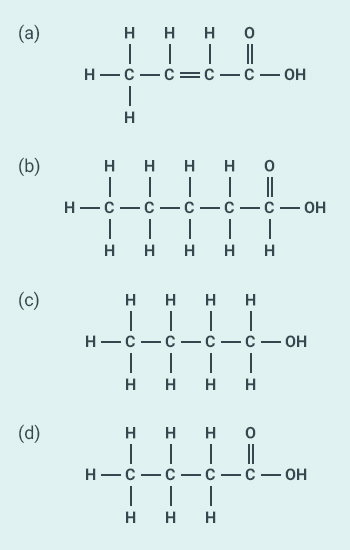
Solution:
d
Question:
19
Vinegar is a solution of:
(a) acetic
acid in alcohol
(b) acetic
acid in alcohol
(c) acetic
acid in water
(d) acetic
acid in water
Solution:
c
Question:
20
Mineral acids are stronger acids than
carboxylic acids because
(i) Mineral acids are completely
ionised
(ii) Carboxylic acids are completely
ionised
(iii) Mineral acids are partially
ionised
(iv) Carboxylic acids are partially
ionised
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Solution:
a
Question:
21
Carbon forms four covalent bonds by
sharing its four valence electrons with four univalent atoms, e.g. hydrogen.
After the formation of four bonds, carbon attains the electronic configuration
of:
(a) Helium
(b) Neon
(c) Argon
(d) Krypton
Solution:
b
Question: 22
The correct electron dot structure of a water
molecule is:
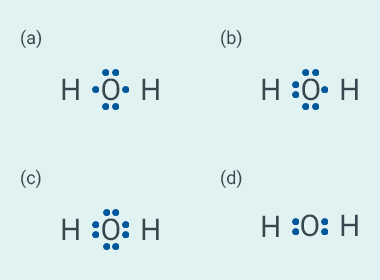
Solution:
c
Question:
23
Which of the
following is not a straight chain hydrocarbon?
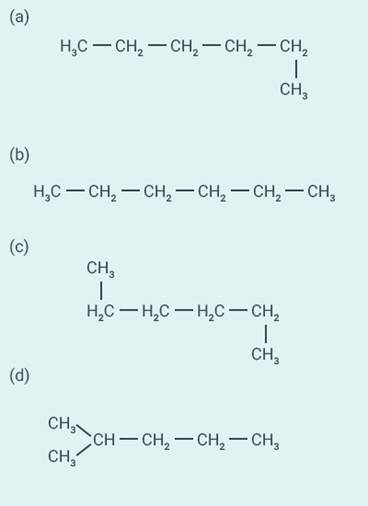
Solution:
d
Question;
24
Which among the following are unsaturated
hydrocarbons?
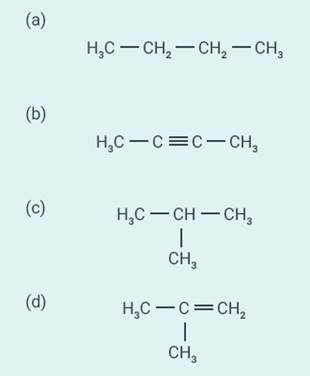
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Solution:
c
Question:
25
Which of the following does not belong to the same
homologous series?
(a)
(b)
(c)
(d)
Solution:
d
Question:
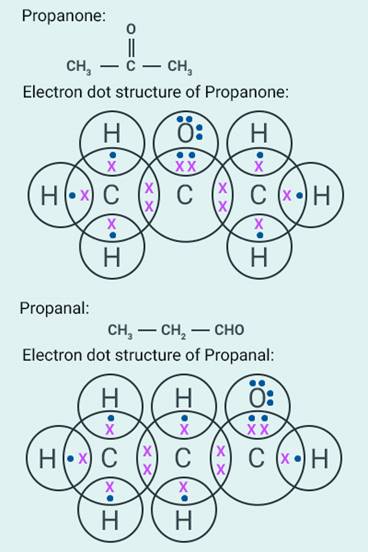
26
The name of
the compound is:
(a) Propanal
(b) Propanone
(c) Ethanol
(d) Ethanal
Solution:
a
Question:
27
The
heteroatoms present in are:
(i) Oxygen
(ii) Carbon
(iii) Hydrogen
(iv) Chlorine
(a) (i) and (ii)
(b) (ii) and
(iii)
(c) (iii) and
(iv)
(d) (i) and
(iv)
Solution:
d
Question:
28
Which of the following represents saponification
reaction?
(a)
(b)
(c)
(d)
Solution:
d
Question:
29
The first member of alkyne homologous series is:
(a) Ethyne
(b) Ethene
(c) Propyne
(d) Methane
Solution:
a
Question:
30
Draw the electron dot structure of ethyne and also
draw its structural formula:
Solution:
Structural formula of ethyne




 H C C H
H C C H
Electron dot structure of ethyne ( )

Question:
31
Write the names of the following compounds:
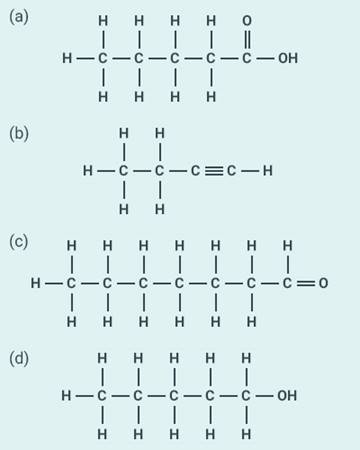
Solution:
(a) Pentanoic
acid
(b) Butyne
(c) Heptanal
(d) Pentanol
Question:
32
Identify
and name the functional groups present in the following compounds.
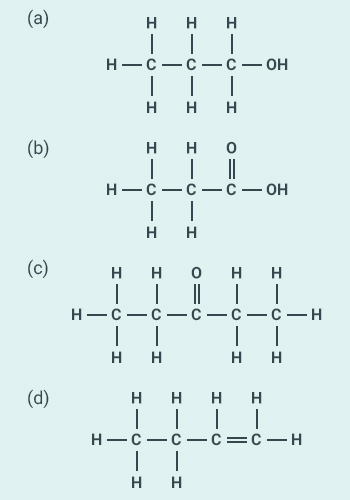
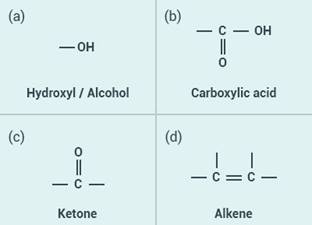
Solution:
Question:
33
A compound X
is formed by the reaction of a carboxylic acid and an alcohol in presence of a few drops of . The alcohol
on oxidation with alkaline followed by acidification gives the same
carboxylic acid as used in this reaction. Give the names and structures of (a)
carboxylic acid, (b) alcohol and (c) the compound X. Also, write the reaction.
Solution:
(a) The carboxylic
acid is ethanoic acid.
(b) The alcohol
is ethanol.
(c) X is ethyl
ethanoate.
The reaction is as follows:
Question:
34
Why detergents are better cleansing agents than
soaps? Explain.
Solution:
Detergents
are better cleansing agents than soaps. This is because detergents are
effective even in hard water whereas, soaps cannot be used in hard water for
washing clothes or cleaning dirt. This is because the charged ends of
detergents do not react with calcium and magnesium ions in hard water to form
insoluble precipitate.
Question:
35
Name the functional
groups present in the following compounds
(a)
(b)
(c)
(d)
Solution:
(a) Ketone
(b) Carboxylic acid
(c) Aldehyde
(d) Alcohol
Question:
36
How is ethene
prepared from ethanol? Give the reaction involved in it.
Solution:
Ethanol
dehydrates on heating with concentrated sulphuric acid at 443 K and gives ethane
and water.
Question:
37
Intake of
small quantity of methanol can be lethal. Give comment.
Solution:
Methanol gets oxidised in the liver and forms methanal.
Methanal reacts rapidly with the protoplasm and leads to its coagulation. It
also blocks the optic nerves leading to blindness.
Question:
38
A gas is
evolved when ethanol reacts with sodium. Name the gas evolved and also write
the balanced chemical equation of the reaction involved.
Solution:
Hydrogen gas is evolved when ethanol reacts with
sodium. The equation for the reaction is:
Question:
39
Ethene is formed when ethanol at 443 K is heated
with excess of concentrated sulphuric acid. What is the role of sulphuric acid
in this reaction? Write the balanced chemical equation of this reaction.
Solution:
When ethanol is heated to form ethane, sulphuric
acid acts as a dehydrating agent in the reaction.
Question:
40
Carbon, Group (14) element in the Periodic Table,
is known to form compounds with many elements.
Write an example of a compound formed with:
(a) Chlorine (Group 17 of Periodic Table)
(b) Oxygen (Group 16 of Periodic Table)
Solution:
(a) Carbon tetrachloride ( )
(b) Carbon dioxide ( )
Question:
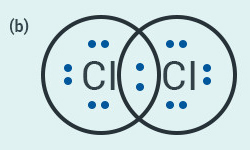
41
In electron dot structure, the valence shell
electrons are represented by crosses or dots.
(a) The atomic number of chlorine is 17. Write its
electronic configuration
(b) Draw the electron dot structure of chlorine
molecule.
Solution:
(a) Electronic configuration of chlorine:
|
|
K
|
L
|
M
|
|
Number of electrons
|
2
|
8
|
7
|
Question:
42
Catenation is the ability of an atom to form bonds
with other atoms of the same element. It is exhibited by both carbon and
silicon. Compare the ability of catenation of the two elements. Give reasons.
Solution:
Carbon has a smaller size. Due to its smaller size,
carbon exhibits catenation much more than silicon, which has a relatively
bigger size. This smaller size makes the CC bonds strong
while the SiSi bonds are
comparatively weaker.
Question:
43
Unsaturated hydrocarbons contain multiple bonds
between the two C-atoms and show addition reactions. Give the test to distinguish
ethane from ethene.
Solution:
Unsaturated hydrocarbons have double or triple
bonds between two carbon atoms. They participate in the addition reaction.
Due to the presence of double bond, these molecules
can break one of the carbon-carbon bonds and can add other atoms by sharing of
electrons with new atoms.
Thus, addition reaction results in the formation of
a saturated compound.
All unsaturated carbon compounds
decolorize bromine water whereas all saturated hydrocarbons do not decolorize
bromine water.
Ethane and ethene can, thus, be distinguished by
the bromine water test.
Question:
44
Match the reactions given in Column (A) with the
names given in column (B).
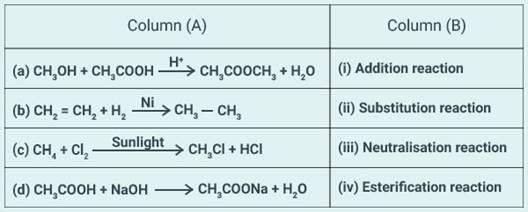
Solution:
(a) (iv)
(b) (i)
(c) (ii)
(d) (iii)
Question:
45
Write the structural formulae of all the isomers of
hexane.
Solution:
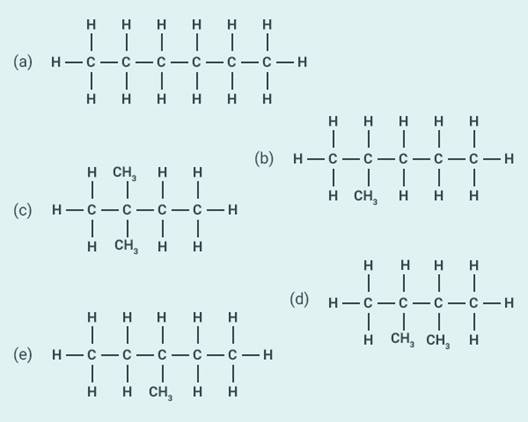
Question:
46
What is the role of metal or reagents written on
arrows in the given chemical reactions?
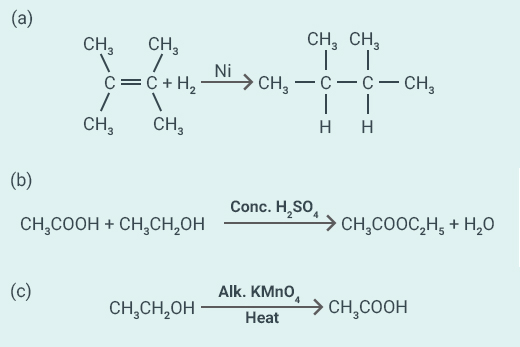
Solution:
(a) Ni acts
as a catalyst.
(b) Concentrated acts as a
catalyst.
(c) Alkaline acts as an
oxidising agent.
Question:
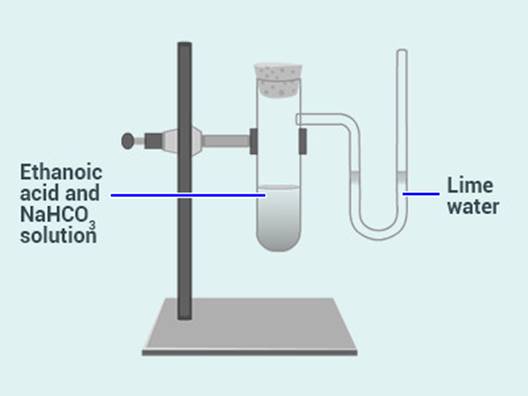
47
A salt X is formed and a gas is evolved when
ethanoic acid reacts with sodium hydrogen carbonate. Name the salt X and the
gas evolved. Describe an activity and draw the diagram of the apparatus to
prove that the evolved gas is the one which you have named. Also, write
chemical equation of the reaction involved.
Solution:
When ethanoic acid reacts with sodium hydrogen carbonate,
sodium ethanoate is formed. Thus, X is sodium ethanoate.
Gas evolved is carbon dioxide.
Activity: The gas that is evolved is passed through
lime water. It turns the lime water milky. This confirms the evolution of
carbon dioxide gas in the reaction.
Question:
48
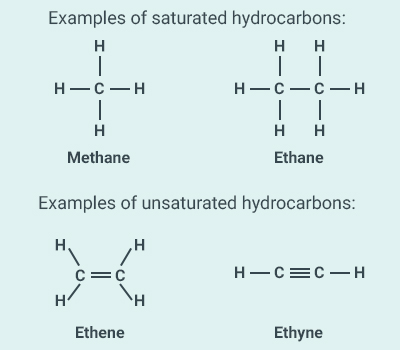
(a) What are hydrocarbons? Give examples.
(b) Give the structural differences between
saturated and unsaturated hydrocarbons with two examples each.
(c) What is a functional group? Give examples of
four different functional groups.
Solution:
(a) Hydrocarbons are compounds formed mainly from
carbon and hydrogen. E.g. methane, ethene, propyne, butanol. pentanoic acid,
etc.
(b) In saturated hydrocarbons, there is no double or
triple bond between two carbon atoms.
In unsaturated hydrocarbons, there is at least one
double or triple bond between two carbon atoms.
(c) Functional group A functional group is an atom or a group of
atoms joined in a specific manner which is responsible for the characteristic
chemical properties of the organic compounds. Examples of functional groups
includes hydroxyl group (OH), aldehyde
group (CHO), carboxylic
group (COOH), etc.
Question:
49
Name the
reaction which is commonly used in the conversion of vegetable oils to fats.
Explain the reaction involved in detail.
Solution:
The reaction which is commonly used in the conversion of vegetable oils to fats is
called hydrogenation reaction.
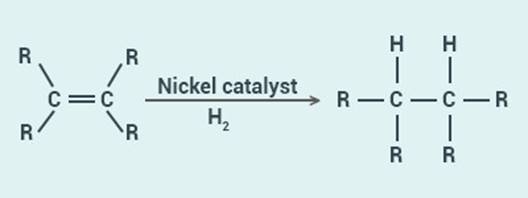
This reaction involves
the addition of hydrogen to an unsaturated hydrocarbon to obtain saturated
hydrocarbon. This reaction is carried out in the presence of a catalyst like
nickel or palladium.
It can be seen that one
of the two bonds between the carbon atoms breaks to accommodate hydrogen and
form saturated hydrocarbon.
Question:
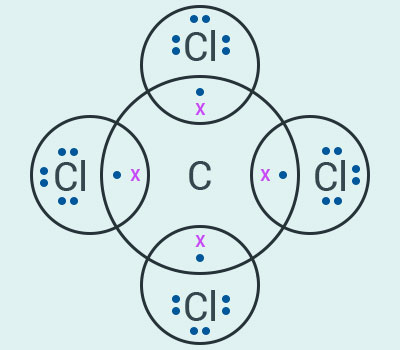
50
(a) Write the formula and draw electron dot
structure of carbon tetrachloride.
(b) What is saponification? Write the reaction
involved in this process.
Solution:
a)
The chemical
formula for carbon tetrachloride is .
(b) Saponification is the process of making soap.
It involves conversion of ethyl acetate into salts of carboxylic acids and
ethanol by treating them with a base.
Question 51
Esters are sweet-smelling substances and are used
in making perfumes. Suggest some activity and the reaction involved for the
preparation of an ester with well labelled diagram.
Solution:
Activity
·
Take
1ml of pure ethanol and 1ml ethanoic acid along with a few drops of
concentrated sulphuric acid in a test tube.
·
Warm
the content in a water-bath at about for
at least 5 minutes.
·
Pour
the content into a beaker that contains 20-50 ml of water and smell the
resulting mixture.
Observation: The mixture smells sweet indicating
the formation of ester.
The reaction is as follows:
Question:
52
A compound C (molecular formula )
reacts with Na metal to form a compound R and evolves a gas
which burns with a pop sound. Compound C on treatment with an alcohol A in the
presence of an acid forms a sweet-smelling compound S (molecular formula ).
On addition of NaOH to C, it also gives R and water. Son treatment with NaOH
solution gives back R and A. Identify C, R, A, S and write down the reactions
involved.
Solution:
Here, compound C (molecular formula )
is ethanoic acid.
R is formed when ethanoic acid reacts with sodium. Thus,
R is sodium salt of ethanoic acid (sodium acetate) and gas evolved is hydrogen.
On reacting with an alcohol A, ethanoic acid forms
a compound S (molecular formula ).
Thus, S is an ester (methyl acetate) and alcohol A
is methanol.
The reactions are:
(a)
(b)
(c)
(d)
Question:
53
Look at the figure which is given below and answer
the following questions.
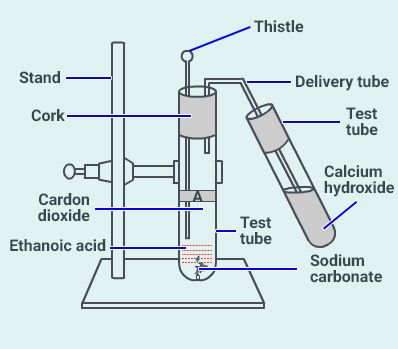
(a) What change would you observe in the calcium
hydroxide solution taken in tube B?
(b) Write the reaction involved in test tubes A and
B respectively.
(c) If ethanol is given instead of ethanoic acid,
would you expect the same change?
(d) How can a solution of limewater be prepared in
the laboratory?
Solution:
(a) Calcium hydroxide solution will turn milky when the
gas evolved in the reaction is passed through it.
(b) Reactions involved in test tube A
Reactions involved in test tube B
(c) Ethanol ( ) does not
react with sodium carbonate ( ). Hence,
a similar change is not expected.
(d) When calcium oxide is dissolved in water and the
supernatant liquid is decanted, lime water is obtained.
Question:
54
How would you bring about the following
conversions?
Name the process and write the reaction involved.
(a) Ethanol to ethene.
(b) Propanol to propanoic acid.
Solution:
(a) By the dehydration of ethanol in the presence of
concentrated ,
(b) By the oxidation of propanol using oxidizing agent
such as alkaline ,
Question:
55
Draw the possible isomers of the compound with
molecular formula and also
give their electron dot structures.
Solution:
Question:
56
Explain the given reactions with the examples:
(a) Hydrogenation reaction
(b) Oxidation reaction
(c) Substitution reaction
(d) Saponification reaction
(e) Combustion reaction
Solution:
(a) Hydrogenation reaction :
The addition of hydrogen to an unsaturated hydrocarbon
to make it saturated is known as hydrogenation reaction.
Example:
(b) Oxidation reaction: The reaction which involves
addition of oxygen to a reactant or removal of hydrogen from a reactant is
called oxidation reaction.
Example:
Methane reacts with oxygen to form carbon dioxide and water. Here
methane is oxidised to carbon dioxide.
(c) Substitution
reaction: When an atom or a group of atoms substitutes another atom or a group
of atoms from the molecule, it is known as substitution reaction.
Example: In the presence of sunlight, chlorine is
added to methane to form methyl chloride and hydrochloric acid.
(d) Saponification: A reaction in which an ester is
hydrolysed in the presence of a base is called saponification reaction.
Example: It is used in
the preparation of soap.
(e)
Combustion reaction: A reaction, in which organic compounds burn readily in air
to form CO2 and water vapour along with lot of heat, is known as
combustion reaction.
Example: Methane burns in air to form carbon
dioxide and water, releasing large amount of heat and light.
Question:
57
An organic compound A on heating with concentrated forms a
compound B which on addition of one mole of hydrogen in the presence of Ni
forms a compound C. One mole of compound C on combustion forms two moles of and 3 moles
of . Identify the compounds A, B and C and write the
chemical equations of the reactions involved.
Solution:
One mole of compound C on combustion forms two
moles of and 3 moles
of . This means that the compound has at least 2
carbon atoms and 6 hydrogen atoms. Thus, the molecular formula of C is (ethane).
Compound C, i.e. ethane, is obtained by the
addition of one mole of hydrogen ( ) to
compound B. Thus, compound B should be (ethene).
Compound B, i.e. ethene, is obtained by heating the
compound A with concentrated which
shows it to be an alcohol. So compound A could be (ethanol).
A is ethanol ( )
B is ethene ( )
C is ethane ( )


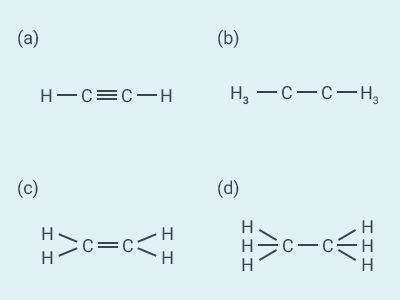





![]()
![]()
![]()
![]()
![]() H C C H
H C C H













