Lesson: Acids, Bases and Salts
Type 1 Very Short Answer Questions (7 Q.)
Question: 1
What is the chemical name of baking soda?
Solution:
Sodium hydrogen carbonate
Question: 2
Name the base which is commonly used in window cleaners.
Solution:
Ammonium hydroxide
Question: 3
Name one antacid which is used to treat indigestion.
Solution:
Milk of magnesia (Magnesium hydroxide)
Question: 4
Name the new substance formed in neutralisation reaction.
Solution:
Salt
Question: 5
Name one synthetic and a natural indicator.
Solution:
Phenolphthalein- synthetic indicator
Litmus paper- natural indicator
Question: 6
Can you name two bases that can be added when soil is acidic in nature?
Solution:
Quick lime (calcium oxide) and slaked lime (calcium hydroxide)
Question: 7
Which acid is present in tomatoes?
Solution:
Citric acid
Type 2 Short Answer Questions (7 Q.)
Question: 1
Why should we take care while handling acids and bases in a laboratory?
Solution:
Care should be taken while handling laboratory acids and bases because these are mostly corrosive in nature, irritating and harmful to the skin.
Question: 2
What is the impact of calamine solution on an ant bite?
Solution:
The calamine solution, which contains zinc carbonate, is basic in nature, which neutralises the effect of the liquid substance (formic acid) injected by the ants through their sting.
Question: 3
What will happen if untreated factory wastes are directly released into water bodies?
Solution:
It is very important to neutralise factory wastes before releasing it into water bodies. Untreated factory wastes contain acids. These are harmful for the organisms living in these water bodies because the acid kills fishes and other organism living in these water bodies.
Question: 4
Litmus solution is used for testing the nature of the substances given below. Complete the table according to your understanding.

Solution:

Question: 5
Why is a solution of baking soda preferred over its solid form for testing its nature?
Solution:
A solution of baking soda is preferred over its solid form because in a solution, the ions are free to move in comparison with the solid form where the ions are bound, and therefore the result of the experiments may differ.
Question: 6
What are salts? What is the nature of salts?
Solution:
An acid and a base react and neutralise each other to form a salt. A salt may be acidic, basic or neutral in nature.
Type 3 Long Answer Questions (5 Q.)
Question: 1
Explain neutralisation with an example from day to day life.
Solution:
Neutralisation reaction is the reaction between an acid and a base. The new substance formed by this reaction is called salt. Salt and water are produced in this process and heat is evolved.
![]()
For example:
In our stomach excessive secretion of hydrochloric acid causes indigestion. Sometimes indigestion is painful. To get relief from indigestion, we take in antacids, such as milk of magnesia, which contains magnesium hydroxide. It neutralises the effect of excessive acid.
Question: 2
Explain the formation of acid rain and its harmful impact.
Solution:
The rain containing excessive amount of acids is known as acid rain. When air pollutants like carbon dioxide, sulphur dioxide and nitrogen dioxide etc. dissolve in raindrops to form carbonic acid, sulphuric acid and nitric acid respectively, the rain becomes acidic. These acids mix with rain and causes harmful impacts, like it can cause damage to buildings, historical monuments, plants and animals.
Question: 3
Explain the effect of all the three indicators- Litmus paper, Turmeric solution and Phenolphthalein on acids and bases.
Solution:
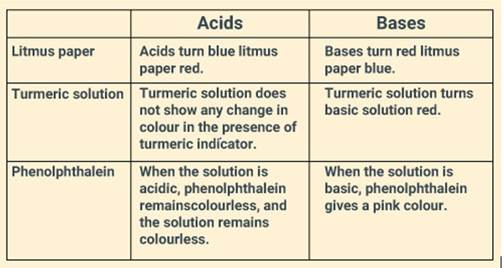
Question: 4
Explain how acids and bases play an important role in soil treatment.
Solution:
We know that the farmers use chemical fertilisers, which help in making the soil fertile but excessive use of chemical fertilisers makes the soil acidic. Plants do not grow well when the soil is too acidic or too basic. When the soil is too acidic, it is treated with bases, like quick lime (calcium oxide) or slaked lime (calcium hydroxide). If the soil is basic, organic matters are added to it. Organic matters release acids which neutralises the basic nature of the soil. Thus, acids and bases play an important role in soil treatment.
Question: 5
Why is toothpaste used for brushing our teeth?
Solution:
When we eat food, the bacteria present in our mouth start acting on it. These bacteria grow on the leftover sugar in our mouth and produces acids which are responsible for tooth decay. If we do not clean our mouth and teeth thoroughly every day, then these bacteria act on the leftover food and over a period of time damages our teeth. Therefore, toothpaste is used to brush our teeth, as it is basic in nature. The basic nature of the toothpaste neutralizes the effects of acids and prevents tooth decay.