Lesson: Acids, Bases and Salts
Question: 1
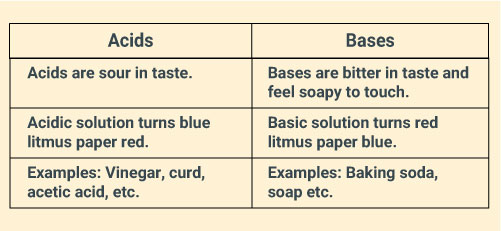
State differences between acids and bases.
Solution:
Question: 2
Ammonia is found in many household products, such as window cleaners. It turns red litmus blue. What is its nature?
Solution:
Ammonia is basic in nature.
Question: 3
Name the source from which litmus solution is obtained. What is the use of this solution?
Solution:
Litmus solution is extracted from lichens. It is the most commonly used natural indicator. It has mauve (purple) colour in distilled water. When added to an acidic solution, it turns red and when added to a basic solution, it turns blue.
Question: 4
Is the distilled water acidic/basic/neutral? How would you verify it?
Solution:
Distilled water is neutral in nature. We can verify this by using litmus paper. Distilled water does not change the colour of litmus paper. This means it is neither acidic nor basic, thus neutral.
Question: 5
Describe the process of neutralisation with the help of an example.
Solution:
The reaction between an acid and a base is known as neutralisation. Salt and water are produced in this process with the evolution of heat.

For example:
Let’s fill one-fourth of a test tube with dilute hydrochloric acid and add a few drops of litmus solution. We observe the colour change in the solution. The solution becomes red. Now, to this acidic solution, gradually add drops of sodium hydroxide solution, drop by drop with a dropper. Stir the tube gently. Continue adding the sodium hydroxide solution drop by drop while stirring, till the colour become green. Now the effect of hydrochloric acid is neutralized by the base sodium hydroxide.
Question: 6
Mark ‘T’ if the statement is true and ‘F’ if it is false:
(i) Nitric acid turns red litmus blue. (T/F)
(ii) Sodium hydroxide turns blue litmus red. (T/F)
(iii) Sodium hydroxide and hydrochloric acid neutralise each other and form salt and water. (T/F)
(iv) Indicator is a substance which shows different colours in acidic and basic solutions. (T/F)
(v) Tooth decay is caused by the presence of a base. (T/F)
Solution:
(i) False
(ii) False
(iii) True
(iv) True
(v) False
Question: 7
Dorji has a few bottles of soft drink in his restaurant. But, unfortunately, these are not labelled. He has to serve the drinks on the demand of customers. One customer wants acidic drink; another wants basic and third one wants neutral drink. How will Dorji decide which drink is to be served to whom?
Solution:
Dorji can decide which drink is acidic or basic or neutral by using a litmus paper. He needs to add few drops of each drink on a litmus paper and if the litmus paper turns red, then the drink is acidic. If it turns blue, it is basic and if it does not change its colour, it is neutral.
Question: 8
Explain why:
(a) An antacid tablet is taken when you suffer from acidity.
(b) Calamine solution is applied on the skin when an ant bites.
(c) Factory waste is neutralised before disposing it into the water bodies.
Solution:
(a) Our stomach contains hydrochloric acid, which helps in digestion. If the amount of acid in the stomach is very high, it causes acidity. In that case, antacid tablets are taken, which are basic in nature. The antacid neutralizes the effect of the excessive acid.
(b) When an ant bites, it injects acetic acidic into the skin, which causes irritation. To neutralise the effect of it, calamine solution is applied which contains zinc carbonate which is basic in nature.
(c) Factory waste generally contains acids. If it is disposed in a water body without neutralisation, the acid will have negative impact on the aquatic life and may even kill the organisms living there. Therefore, prior disposal, factory waste is treated with a base.
Question: 9
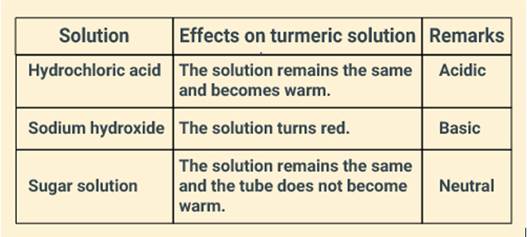
Three liquids are given to you. One is hydrochloric acid; another is sodium hydroxide and third is a sugar solution. How will you identify them? You have only turmeric indicator.
Solution:
Turmeric is a natural indicator which is yellow in colour. When a base is added to it, the solution turns red. However, turmeric remains yellow when an acid or neutral solution is added to it. So, in order to identify the liquids, we can perform a test, from which we can infer the nature of the given solutions.
(i) Take three test tubes and add a few drops of the given solution in each of the test tubes separately.
(ii) Now add a few drops of the turmeric solution in each of the test tubes. If the solution turns red, the solution is basic.
(iii) Now add a few drops of this basic solution into the second test tube, and then add turmeric solution into it. If the colour does not change and the test tube becomes warm because of neutralisation reaction, it would mean the solution is acidic, otherwise neutral.
The inference of the test can be tabulated, as given below:
Question: 10
Blue litmus paper is dipped in a solution. It remains blue. What is the nature of the solution? Explain.
Solution:
If a blue litmus paper is dipped in a solution and it remains blue, it indicates that either is the solution basic, or is it neutral in nature.
Question: 11
Consider the following statements:
(a) Both acids and bases change colour of all indicators.
(b) If an indicator gives a colour change with an acid, it does not give a change with a base.
(c) If an indicator changes colour with a base, it does not change colour with an acid.
(d) Change of colour in an acid and a base depends on the type of the indicator.
Which of these statements are correct?
(i) All four
(ii) a and d
(iii) b and c
(iv) only d
Solution:
(iv)