Lesson: Heat
Question: 1
State similarities and differences between the laboratory thermometer and the clinical thermometer.
Solution:
Similarities:
a) Both the thermometers have a long narrow glass tube
b) Both the thermometers have mercury to measure the temperature.
c) Both the thermometers have a bulb at one end.
Differences:
a) A clinical thermometer is used to only measure temperature of a human body, whereas laboratory thermometer is used to measure temperature of different objects in a laboratory.
b) In a laboratory thermometer kink is absent, whereas in a clinical thermometer kink is present.
c) The range of a laboratory thermometer is more than the range of a clinical thermometer.
d) Laboratory thermometer is required to be kept straight while measuring the temperature whereas clinical thermometer can be tilted while measuring the temperature.
Question: 2
Give two examples each of conductors and insulators of heat.
Solution:
Conductors: Copper, Aluminium
Insulator: Air, Glass, Wood
Question: 3
Fill in the blanks:
a) The hotness of an object is determined by its _______.
b) Temperature of boiling water cannot be measured by a _______ thermometer.
c) Temperature is measured in degree _______.
d) No medium is required for transfer of heat by the process of _______.
e) A cold steel spoon is dipped in a cup of hot milk. It transfers heat to its other end by the process of _______ _ .
f) Clothes of ______ colours absorb heat better than clothes of light colours.
Solution:
(a) The hotness of an object is determined by its temperature.
(b) Temperature of boiling water cannot be measured by a clinical thermometer.
(c) Temperature is measured in degree Celsius.
(d) No medium is required for transfer of heat by the process of radiation.
(e) A cold steel spoon is dipped in a cup of hot milk. It transfers heat to its other end by the process of conduction.
(f) Clothes of dark colours absorb heat better than clothes of light colours.
Question: 4
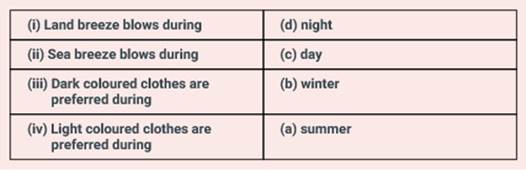
Match the following:
|
(i) Land breeze blows during |
(a) summer |
|
(ii) Sea breeze blows during |
(b) winter |
|
(iii) Dark coloured clothes are preferred during |
(c) day |
|
(iv) Light coloured clothes are preferred during |
(d) night |
Solution:
Question: 5
Discuss why wearing more layers of clothing during than winter keeps us warmer wearing just one thick piece of clothing.
Solution:
Air is a bad conductor of heat. Wearing more layers of clothing, traps more air between the layers of the clothes. This trapping of air prevents our body heat to escape outside as a result we do not feel cold. Therefore, wearing more layers of clothing during winter keeps us warmer than wearing just one thick piece of clothing.
Question: 6
Look at the figure given below. Mark where the heat is being transferred by conduction, by convection and by radiation.
Solution:
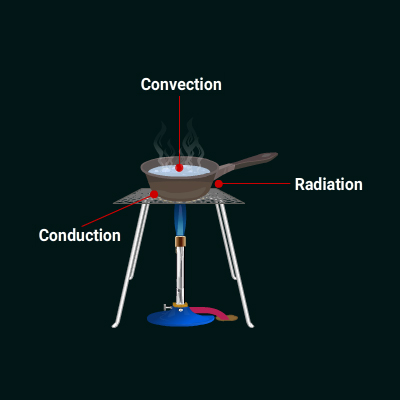
a. Water in the pan gets heated through convection.
b. Heat is transferred by the hot plate to the pan by conduction.
c. Heat is transferred to surrounding air through radiation.
Question: 7
In places of hot climate, it is advised that the outer walls of houses be painted white. Explain.
Solution:
In places of hot climate, it is advised that the outer walls of houses be painted white, because white colour is a good reflector of heat. White colour reflects most of the light that falls on it and therefore absorbs less amount of heat. Therefore, temperature inside the houses does not increase too much and the houses remain cool.
Question: 8
One litre of water at is mixed with one litre of water at . The temperature of the mixture will be
(a) (b) more than but less than
(c) (d) between and
Solution:
(d) Between and
Question: 9
An iron ball at is dropped in a mug containing water at .
The heat will
(a) Flow from iron ball to water.
(b) Not flow from iron ball to water or from water to iron ball.
(c) Flow from water to iron ball.
(d) Increase the temperature of both.
Solution:
(b) Not flow from iron ball to water or from water to iron ball because both have same temperature.
Question: 10
A wooden spoon is dipped in a cup of ice cream. Its other end
(a) Becomes cold by the process of conduction.
(b) Becomes cold by the process of convection.
(c) Becomes cold by the process of radiation.
(d) Does not become cold.
Solution:
(d) Does not become cold. This is because wood is an insulator.
Question: 11
Stainless steel pans are usually provided with copper bottoms. The reason for this could be that
(a) Copper bottom makes the pan more durable.
(b) Such pans appear colourful.
(c) Copper is a better conductor of heat than the stainless steel.
(d) Copper is easier to clean than the stainless steel.
Solution:
(c) Copper is a better conductor of heat than stain less steel.