Lesson: Acid, Bases and Salts
Multiple Choice Questions
The correct way of making a solution of acid in water is to:
(a) Add water to acid.
(b) Add acid to water.
(c) Mix acid and water simultaneously.
(d) Add water to acid in a shallow container.
Solution:
(b)
Question: 2
Products of a neutralisation reaction are always
(a) An acid and a base
(b) An acid and a salt
(c) A salt and water
(d) A salt and a base
Solution:
(c)
Question: 3
Turmeric is a natural indicator. On adding its paste to acid and base separately, which colours would be observed
(a) Yellow in both acid and base.
(b) Yellow in acid and red in base.
(c) Pink in acid and yellow in base.
(d) Red in acid and blue in base.
Solution:
(b)
Question: 4
Phenolphthalein is a synthetic indicator and its colours in acidic and basic solutions, respectively are
(a) Red and blue.
(b) Blue and red.
(c) Pink and colourless
(d) Colourless and pink
Solution:
(d)
Question: 5
When the soil is too basic, plants do not grow well in it. To improve its quality what must be added to the soil?
(a) Organic matter
(b) Quick lime
(c) Slaked lime
(d) Calamine solution
Solution:
(a)
Question: 6
‘Litmus’, a natural dye is an extract of which of the following?
(a) China rose (Gudhal)
(b) Beetroot
(c) Lichen
(d) Blue berries (Jamun)
Solution:
(c)
Question: 7
Neutralisation reaction is a:
(a) Physical and reversible change
(b) Physical change that cannot be reversed
(c) Chemical and reversible change
(d) Chemical change that cannot be reversed
Solution:
(d)
Question: 8
A solution changes the colour of turmeric indicator from yellow to red. The solution is:
(a) basic
(b) acidic
(c) neutral
(d) either neutral or acidic
Solution:
(a)
Question: 9
Which of the following set of substances contain acids?
(a) Grapes, lime water
(b) Vinegar, soap
(c) Curd, milk of magnesia
(d) Curd, vinegar
Solution:
(d)
Question: 10
On adding phenolphthalein indicator to a colourless solution, no change is observed. What is the nature of this solution?
(a) Basic
(b) Either acidic or basic
(c) Either acidic or neutral
(d) Either basic or neutral
Solution:
(c)
Question: 11
Which of the following is an acid-base indicator?
(a) Vinegar
(b) Lime water
(c) Turmeric
(d) Baking soda
Solution:
(c)
Very Short Answer Questions
Question: 12
Look at the given reaction.
Hydrochloric acid + Sodium hydroxide (base) Sodium chloride (salt) + Water
Sodium chloride formed in this reaction remains in solution form. Can we get solid sodium chloride from this solution? Suggest a method (if any).
Solution:
Solid sodium chloride can be obtained from the solution by the process of evaporation. When we boil this solution, water will evaporate and solid form of sodium chloride will be left behind.
Question: 13
State whether the following statements are true or false. Correct the false statements.
(a) All substances are either acidic or basic.
(b) A compound if acidic will turn all indicators red.
(c) Lime water turns red litmus blue.
(d) Common salt dissolved in water turns blue litmus red.
(e) Phenolphthalein is a natural indicator.
(f) Calamine can be used to treat ant’s sting.
(g) Lemon water is basic in nature.
Solution:
(a) False
All substances are either acidic or basic or neutral.
(b) False
A compound if acidic may turn some of its indicators red but otherwise, the colour change depends upon the nature of the indicator.
(c) True
(d) False
Common salt solution does not change the colour of litmus at all.
(e) False
Phenolphthalein is an artificial indicator.
(f) True
(g) False
Lemon water is acidic in nature.
Question: 14
Paheli is suffering from indigestion due to acidity. Is it advisable to give her orange juice in this situation and why?
Solution:
It is not at all advisable to give her orange juice in indigestion, as it is acidic in nature. In case of indigestion due to acidity, excess amount of hydrochloric acid is secreted by the stomach, so generally antacids, which are basic in nature, are advised to be given, to neutralise the acid.
Short Answer Questions
Question: 15
Look at the figure below which shows solutions taken in test tubes A, B, C and D. What colour is expected when a piece of red litmus paper is dropped in each test tube? Nature of the solutions is given in the table for your help.
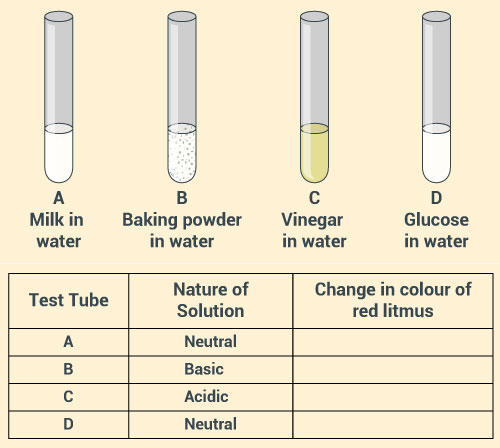
Solution:
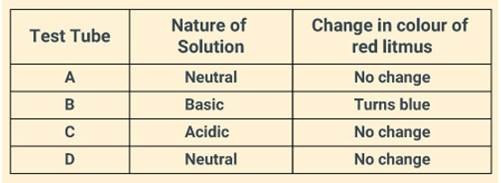
Question: 16
While playing in a park, a child was stung by a wasp. Some elders suggested applying paste of baking soda and others lemon juice as remedy. Which remedy do you think is appropriate and why?
Solution:
Baking soda is an appropriate remedy because during a wasp sting, the wasps inject the acidic liquid (formic acid) into the skin. The effect of this acid can be neutralised by rubbing moist baking soda (sodium hydrogen carbonate).
Question: 17
Form a sentence using the following words baking soda, ant bite, moist, effect, neutralised, and rubbing.
Solution:
The effect of ant bite can be neutralised by rubbing moist baking soda.
Question: 18
Match the substances in Column I with those in Column II.
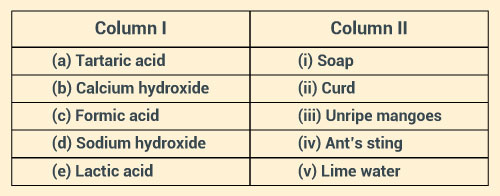
Solution:
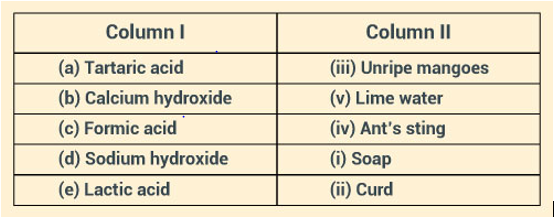
Question: 19
Fill the blanks in the following sentences
(a) Lemon juice and vinegar taste ___________ because they contain ___________.
(b) Turmeric and litmus are _________ acid-base indicators.
(c) Phenolphthalein gives _________ colour with lime water.
(d) When an acidic solution is mixed with a basic solution, they _________ each other forming _________ and water.
Solution:
(a) Sour, acid
(b) Natural
(c) Pink
(d) Neutralise, salt
Long Answer Questions
Question: 20
Boojho, Paheli and their friend Golu were provided with a test tube each containing China rose solution which was pink in colour. Boojho added two drops of solution ‘A’ in his test tube and got dark pink colour. Paheli added 2 drops of solution ‘B’ to her test tube and got green colour. Golu added 2 drops of solution ‘C’ but could not get any change in colour. Suggest the possible cause for the variation in their results.
Solution:
China rose is a natural indicator which turns acidic solutions to dark pink (magenta) and basic solutions to green in colour. From this we can infer the possible cause of variation in their results, here
· Solution ‘A’ is acidic.
· Solution ‘B’ is basic.
· Solution ‘C’ is neutral.
Question: 21
Fill in the crossword given below with the help of the clues provided.
Across
(2) The solution which does not change the colour of either red or blue litmus.
(4) Phenolphthalein gives pink colour in this type of solution.
(7) Colour of blue litmus in lemon juice.
Down
(1) It is used to test whether a substance is acidic or basic.
(3) It is a natural indicator and gives pink colour in basic solution.
(5) Nature of ant’s sting.
(6) It is responsible for increase in temperature during a neutralisation reaction.
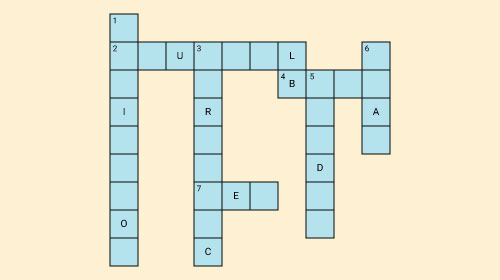
Solution:
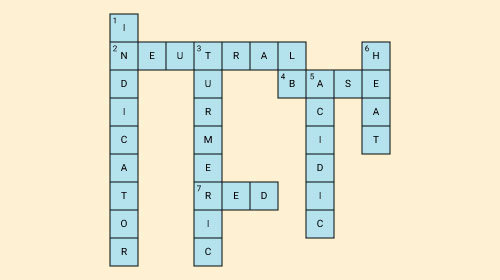
Question: 22
A farmer was unhappy because of his low crop yield. He discussed the problem with an agricultural scientist and realized that the soil of his field was either too acidic or too basic. What remedy would you suggest the farmer to neutralize the soil?
Solution:
If the soil is too acidic, it is treated with bases, like quick lime (calcium oxide) or slaked lime (calcium hydroxide). If the soil is basic, organic matter is added to it. Organic matter releases acids which neutralises the basic nature of the soil. Since the soil is either too acidic or too basic, so we can suggest the farmer to add a mixture of both, in order to neutralise the soil.
Question: 23
You are provided with four test tubes containing sugar solution, baking soda solution, tamarind solution, salt solution. Write down an activity to find the nature (acidic/basic/neutral) of each solution.
Solution:
We can identify the nature of the given solutions using litmus papers which are natural indicators of acid-bases.
The inference of the test can be tabulated, as given below:
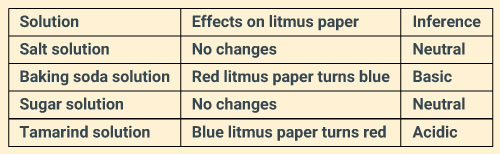
Question: 24
You are provided with three test tubes A, B and C as shown in the figure below with different liquids. What will you observe when you put:
(a) a piece of blue litmus paper in each test tube.
(b) a piece of red litmus paper in each test tube.
(c) a few drops of phenolphthalein solution to each test tube.
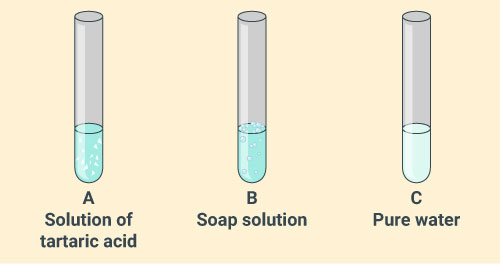
Solution:
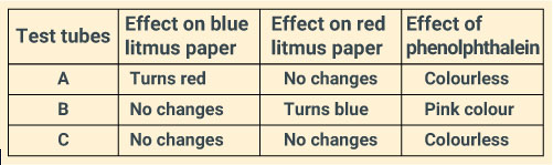
Question: 25
Paheli observed that most of the fish in the pond of her village were gradually dying. She also observed that the waste of a factory in their village is flowing into the pond which probably caused the fish to die.
(a) Explain why the fish were dying.
(b) If the factory waste is acidic in nature, how can it be neutralised?
Solution:
(a) The wastes of many factories mostly contain acids. If they are allowed to flow into the water bodies directly without any prior treatment, then the acids will kill fishes. That is why the fishes were dying in the village.
(b) If the factory waste is acidic in nature it can be neutralised by adding basic substances to it.
Question: 26
Explain two neutralisation reactions related to daily life situation.
Solution:
Two neutralisation reactions related to daily life situation are:
(a) Indigestion- To neutralise the effect of indigestion or acidity, an antacid, such as milk of magnesia, which contains magnesium hydroxide is used. Antacids are basic in nature and they neutralise the effect of excessive hydrochloric acid secreted in our body during indigestion.
(b) Wasp bite- When a wasp bites, it releases a quantity of acidic liquid (formic acid) in the skin which causes irritation. This effect is neutralised by rubbing moist baking soda (sodium hydrogen carbonate) on the skin.